Chlamydia Trachomatis NAAT Culture
| Test Name |
Chlamydia trachomatis NAAT |
|---|---|
| Alternate Name(s) | (Information Unavailable) |
| Laboratory Module |
Microbiology |
| Ordering Mnemonic | CHLAM |
| Specimen Type |
First-void urine, Endocervical or cervix, Female Vaginal, Rectal, Pharyngeal |
| Collection Container |
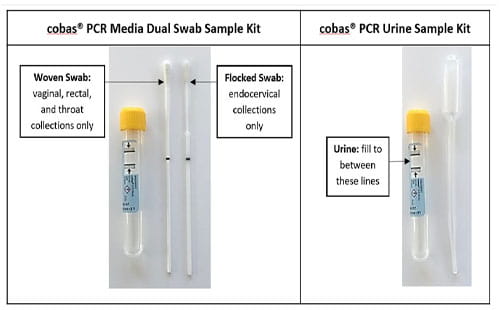
Endocervical/Urethral swab:) Roche cobas® PCR Media Dual Swab Sample Kit Urine: Roche cobas® Urine Sample Kit |
| Container Information |
Unisex swab Urine |
| Collection Information |
Note:Medico-legal investigations: CT and NG culture is the preferred and recommended method for medico-legal investigations; however, NAAT specimens will also be accepted. A positive NAAT result will be confirmed by another NAAT using a different set of primers as per the current Public Health Agency of Canada (PHAC) Canadian Guidelines on Sexually Transmitted Infections. Specimens received on patients <14 years of age have not been validated by the manufacturer; however, they will be tested by PHO with a disclaimer added.
Collect approximately 10-50 mL of first-void urine in a sterile container. Using the provided disposable pipette, transfer urine from the sterile container to the Roche cobas® PCR Urine Sample Kit until the fluid level is between the two black fill lines. Tightly re-cap the Roche cobas® PCR Urine sample tube, and invert 5 times to mix. Urine specimens must be transferred into the Roche cobas® PCR Urine sample tube immediately for stabilization.
The Roche cobas® PCR Media Dual Swab Sample Kit contains two swabs: a flocked swab and a woven swab. Each swab is optimized and validated for testing of a specific genital/extragenital site. The flocked swab is used for endocervical specimens, and the woven swab is used for vaginal, rectal and pharyngeal specimens. Do not pre-wet swabs in Roche cobas® PCR Media or any other liquid before collection of any specimens. Primary swab specimen tubes with no swabs or with two swabs have not been collected according to the collection instructions and therefore will not be tested.
The presence of mucous in endocervical specimens may cause process delays. Mucous-free specimens are required for optimal test performance. For endocervical specimen collection, use the large woven swab provided in the Roche cobas® PCR Media Dual Swab Sample Kit or equivalent device to clean and remove excess mucous. Discard this swab after use. Use only the flocked swab for specimen collection. Specimens received with two swabs in the sample kit will be cancelled. Vaginal, rectal and pharyngeal specimens should be collected using the woven swab. Do not let the woven swab touch any surface before placing it into the sample kit. Do not use the flocked swab; these specimens will be cancelled. |
| Test Schedule |
Send-Out Monday thru Friday |
| Routine Turnaround Time | Preliminary: 48 hours Final: 7 days |
| Stat Turnaround Time | Preliminary: 24 hours Final: 7 days |
| Reference Interval | (Information Unavailable) |
| Critical Values | Critical positive reports are communicated |
| Lab Process Notes | (Information Unavailable) |
| Storage and Transport |
If the specimen cannot be transported directly to the laboratory, the swab should remain at ambient temperature and the urine should be placed in a fridge. Once in the laboratory the swab specimen should be stored at room temperature until processing and the urine should be placed in the fridge. |
| Test Referred To |
Public Health Laboratories, Hamilton |